Abstract
Introduction: Patients with hematologic malignancies are at high risk of severe illness and increased mortality from SARS-CoV-2 infection. Autologous (auto) and allogeneic (allo) hematopoietic stem cell transplant (HCT) recipients also have a higher likelihood of developing severe COVID-19 with increased risk of mortality as described in registry analysis (Sharma et al, Lancet Haematol 2021, Ljungman et al, Leukemia 2021). Several monoclonal antibody (MOAB) combinations, as well as the antiviral remdesivir (Rem) have been approved under an emergency use authorization (EUA) and for outpatients with mild to moderate COVID-19 at risk of progression. There is limited data on HCT patient outcomes in the era of novel COVID-19 directed treatments. The aim of this study is to describe the effectiveness of outpatient (OP) MOABs and Rem in HCT recipients at our institution.
Methods: We conducted a retrospective analysis of HCT patients treated at H. Lee Moffitt Cancer Center who were COVID-19 PCR positive between June 2020 and February 2022. The primary endpoint was hospitalization related to severe SARS-CoV-2 infection. We used Fisher's exact test, the Wilcoxon test and the Chi-squared test to compare the characteristics of patients who progressed to severe COVID-19 requiring hospitalization and those who did not.
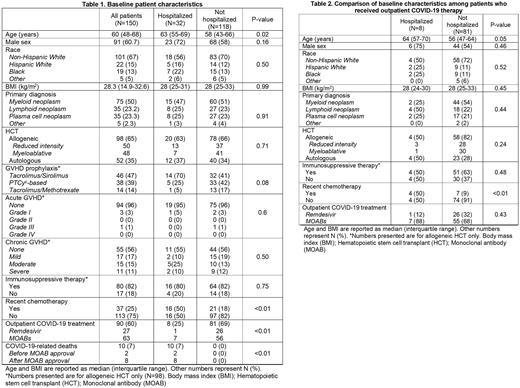
Results: 150 HCT patients were PCR positive for COVID-19 thus were included in this analysis. Patient characteristics are described in Table 1. The mean age was 60 years old (interquartile range [IQR], 48-68 years old) with 61% males. The majority of patients (65%) were recipients of alloHCT, while 35% had autoHCT. Median time from cell therapy to COVID-19 diagnosis was 548 days (IQR 167-1368 days). Data on COVID-19 vaccinations was missing in the majority of patients and was not included in this analysis. At the time of diagnosis, 82% of alloHCT patients were on immunosuppressive therapy (IST) and 25% had received chemotherapy within the past 30 days prior to diagnosis. With a median follow up of 132 days (range 1-875 days), 32 patients (21%) required hospitalization with moderate to severe COVID-19 as primary diagnosis.
A total of 89 subjects (59%) received MOABs active against circulating variants and/or Rem. Sixty-two (70%) were treated with only MOAB and 27 (30%) with Rem. Of those requiring hospitalization, eight (25%) had received OP treatment with MOAB or Rem in comparison to twenty-four patients (75%) who did not receive OP therapy (p-value<0.01). Hospitalized patients who received OP treatment were more likely to be older (median age 64 years [IQR 57-70] vs 56 years [IQR 47-64]; p-value=0.05), male (75% vs 44%; p-value = 0.45) and to have received chemotherapy in the preceding 30 days (50% vs 9%; p-value < 0.01). No differences in race, body mass index (BMI), type of transplant or conditioning were noted (Table 2). Overall, rates of COVID-19 related death were significantly lower in patients who had received OP therapy (N=2 [2.3%]) compared to those who had not (N=8 [13.1%]; p-value = 0.02). Of those admitted, 22 (69%) received inpatient Rem for five days with 59% and 27% receiving concurrent dexamethasone or tocilizumab respectively. Ten hospitalized patients died of COVID-19-related complications resulting in a mortality rate of 31.2% in patients requiring hospitalization.
Conclusions: Treatment with anti-SARS-CoV-2 MOABs and Rem in 89 HCT patients at our institution demonstrated encouraging safety and efficacy in a high-risk population with an overall COVID-19 mortality rate of 2.3% which compares favorably to published data. Despite novel therapies for the treatment of COVID19, further studies are needed in HCT recipients as nearly one third of patients with severe disease requiring hospitalization died. While the study is limited by retrospective design and the presence of confounders, our findings support continued vigilance against COVID-19 in HCT recipients with early testing via PCR swabs and prompt outpatient therapy in mildly symptomatic patients.
Disclosures
Alsina:BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding. Bejanyan:Medexus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDX Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Freeman:Incyte: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria, Research Funding; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hansen:OncLive: Honoraria; BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; Survivorship: Honoraria. Jain:BMS: Consultancy; MyeloidTx: Consultancy; Incyte: Research Funding; Novartis: Consultancy; Kite Pharma: Consultancy, Research Funding. Lazaryan:AvroBio: Consultancy; AmWel: Current equity holder in publicly-traded company; Sanofi: Consultancy; Humanigen: Consultancy; Teladoc: Current equity holder in publicly-traded company. Liu:Sanofi: Speakers Bureau. Locke:Society for Immunotherapy of Cancer: Other: Education or editorial role; BioPharma Communications CARE Education: Other: Education or editorial role; ASH: Other: Education or editorial role; Calibr: Membership on an entity's Board of Directors or advisory committees; Gerson Lehrman Group: Consultancy; Aptitude Health: Other: Education or editorial role; Moffitt Cancer Center: Patents & Royalties: several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; EcoR1: Consultancy; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Imedex: Other: Education or editorial role; BlueBird Bio: Research Funding; Emerging Therapy Solutions: Consultancy; Cowen: Consultancy; Cellular Biomedicine Group: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GammaDelta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Umoja: Membership on an entity's Board of Directors or advisory committees; Wugen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Sana: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Clinical Care Options Oncology: Other: Education or editorial role; Kite Pharma: Research Funding; Novartis: Research Funding; Allogene: Research Funding; BMS: Research Funding; National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Iovance: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; A2: Membership on an entity's Board of Directors or advisory committees. Pidala:Pharmacyclcis: Research Funding; Johnson and Johnson: Research Funding; Takeda: Research Funding; Novartis: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; BMS: Research Funding. Faramand:Novartis: Research Funding; Kite/Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal